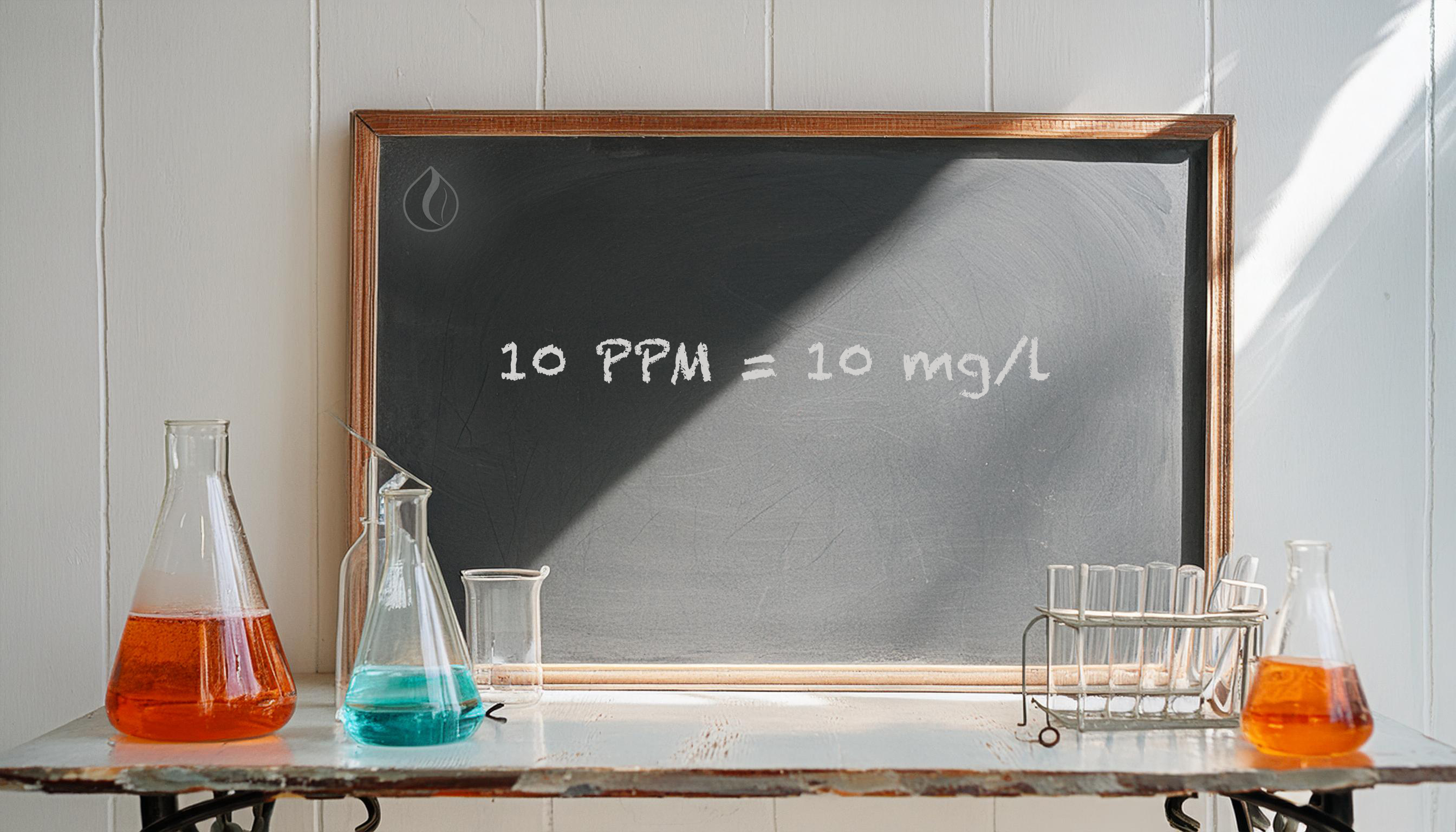
Welcome to Meditech Europe! In this comprehensive blog, we explain how many mg/l are in a colloidal solution. We detail how to calculate the concentration and the impact of particle mass on the total mass of the solution. We provide specific examples of colloidal silver, gold, zinc, and magnesium and highlight how Meditech Europe ensures precise quality control of its products.
What Is a Colloidal Solution?
A colloidal solution is a mixture where microscopic particles of a substance (such as silver, gold, zinc, or magnesium) are evenly dispersed in a liquid, usually water. These particles are so small that they remain suspended without settling.
Terms and Units
Let’s start with some important terms and units relevant to colloidal solutions:
Concentration: Expressed in mg/l (milligrams per liter), it indicates how many milligrams of a substance are in one liter of liquid.
PPM (Parts Per Million): A unit that indicates concentration. 1 PPM means there is one part of a substance per million parts of the total solution.
Conversion Formula: mg/l to PPM
Because the density of water (and thus the liquid) is approximately 1 g/ml, we can assume:
1 mg/l = 1 PPM
This means that if you know the concentration of a colloidal solution in mg/l, you can directly convert it to PPM, and vice versa.
The Mass of Particles and Their Impact on Total Mass
Although water has a density of 1 g/ml, adding colloidal particles changes the total mass of the liquid. The molecular mass of the particles plays an important role here. One liter of water with a certain concentration of colloidal particles will weigh slightly more than one liter of pure water.
Examples of Colloidal Solutions
Let’s look at some specific examples, including their mg/l values:
10 PPM Colloidal Ionic Silver
Colloidal ionic silver with a concentration of 10 PPM means there are 10 milligrams of silver particles per liter of water. Since the density of silver is approximately 10.49 g/cm³, this slightly increases the mass of the liquid, although the difference is minimal.
Concentration: 10 mg/l
5 PPM Colloidal Nano Silver
The 5 PPM colloidal nano silver contains 5 milligrams of silver particles per liter of water. Nano particles have a higher surface/volume ratio, which can affect their properties without significantly increasing the mass.
Concentration: 5 mg/l
5 PPM Colloidal Nano Gold
For 5 PPM colloidal nano gold, there are 5 milligrams of gold per liter of water. The density of gold is significantly higher (approximately 19.32 g/cm³), which has a slightly larger impact on the total mass of the liquid.
Concentration: 5 mg/l
20 PPM Colloidal Zinc
With a concentration of 20 PPM colloidal zinc, there are 20 milligrams of zinc per liter of water. The density of zinc is approximately 7.14 g/cm³, which also increases the mass of the solution somewhat.
Concentration: 20 mg/l
20 PPM Colloidal Magnesium
Finally, 20 PPM colloidal magnesium means there are 20 milligrams of magnesium per liter of water. Magnesium has a density of approximately 1.74 g/cm³, increasing the mass of the liquid slightly, but not as significantly as heavier metals like gold or silver.
Concentration: 20 mg/l
Mass vs. Weight of a Liquid
It’s important to understand the difference between the mass of a liquid and the weight of a liquid:
Mass: Mass is a measure of the amount of matter in an object and is expressed in kilograms (kg) or grams (g). The mass of a liquid remains constant, regardless of its location.
Weight: Weight is the force exerted on an object by gravity. It is expressed in Newtons (N). The weight of a liquid can vary depending on gravity. On Earth, an object with a mass of 1 kg has a weight of approximately 9.81 N.
In the context of colloidal solutions, this means that the mass of the particles increases the total mass of the liquid, but the weight of the liquid can vary depending on gravity.
Bioavailability
The bioavailability of a colloidal or nano variant of a substance can be much higher than that of the regular form due to various physicochemical and biological factors. Here are some reasons why this might be the case:
Increased Surface Area: Nano and colloidal particles have a much larger surface area per unit mass compared to larger particles of the same substance. This increased surface area can lead to enhanced interaction with biological membranes, making the uptake into the body more efficient.
Improved Solubility: Nanoparticles can increase the solubility of hydrophobic substances. In their nano or colloidal form, these substances can be better dispersed in biological fluids, resulting in improved absorption.
Faster Dissolution Kinetics: The rate at which nanoparticles dissolve is often higher than that of larger particles. This can lead to faster and more complete absorption of the substance in the digestive system.
Increased Permeability: Nanoparticles can pass biological barriers such as cell membranes, the intestinal wall, and the blood-brain barrier more easily than larger particles. This can result in increased uptake of the substance into the bloodstream and tissues.
Stabilization of Sensitive Substances: Some substances are sensitive to degradation in the stomach or intestines. Nanoparticles can protect these substances from enzymatic or chemical breakdown, allowing them to reach the absorption sites intact.
Active Targeting: Nanoparticles can be designed to target specific cells or tissues, for example by adding ligands that bind to receptors on the target cells. This can lead to an increased local concentration of the substance at the desired site of action.
Controlled Release Profile: Colloidal and nanoparticles can be designed to provide a controlled release of the active substance. This can ensure a more constant plasma concentration and an extended duration of action, contributing to improved bioavailability.
Reduced First-Pass Metabolism: Nanoparticles can bypass first-pass metabolism in the liver by delivering the substance directly into the systemic circulation, thereby increasing bioavailability.
Quality Control at Meditech Europe
At Meditech Europe, we conduct extensive research into the specifications of our colloidal products. Our solutions are constantly and precisely tested during and after production to ensure consistency and quality. Through these rigorous processes, we can guarantee that our products meet the highest standards and are effective for our customers.
Conclusion
Calculating the concentration of colloidal solutions in mg/l is relatively simple thanks to the direct relationship between mg/l and PPM. The mass of the particles plays a role in the total mass of the liquid, which is important for accurate measurements and applications. At Meditech Europe, we are committed to delivering the highest quality colloidal products through careful research and constant quality control.
Do you have questions about our products or want more information about colloidal solutions? Feel free to contact us!









